Background
Miniaturized devices have had a significant impact on our society in revolutionizing the electronics industry. A similar trend of size-reduction is emerging in the health care industry to produce small bioanalytical devices for disease diagnosis, novel methods for drug delivery to treat diseases, and microfabricated platforms for studying microorganisms. Nanostructured materials, in particular, have revolutionized capabilities of biotic-abiotic interfaces in various biomedical devices, including affinity biosensors, implantable neural electrodes, and drug delivery platforms. Despite the recent research on these materials, significant challenges remain in controlling material properties, interfacing nanocomponents with instrumentation, and engineering their interaction with biological systems.
Prior to joining UC Davis as a faculty member, Seker’s dissertation focused on microfabrication and thermo-mechanical characterization of nanoporous gold1,2, a promising candidate for functional surface coatings due to its controllable morphology, electrical conductivity, and well-studied gold surface chemistry3. In addition, he studied mass-transport in porous media4 and synthesized a composite material that exhibits very low elastic modulus and high electrical conductivity5 – highly desirable attributes for flexible electrodes. As a postdoctoral researcher in chemistry department, his research extended to the study of biomolecule-surface interactions6 and development of novel flow control methods in microfluidic circuits7,8. As a research associate at Center for Engineering in Medicine (CEM), he began to utilize nanoporous metals for developing planar multiple electrode arrays for electrophysiology applications9.
The overarching objective of our group is to utilize our expertise at the intersection of nanoporous metal synthesis, microfluidics, and device engineering to overcome challenges in the evolution of miniaturized devices relevant to microelectronics and life sciences. We gratefully acknowledge support from National Science Foundation (1512745 & 1454426), UC Lab Fees Research Program (12-LR-237197), NIH Biotechnology Training Program (T32-GM008799), and UC Davis Research Investments in the Sciences and Engineering (RISE).
The projects listed below are a selected group of our current research thrusts. Please contact Prof. Erkin Şeker for more information.
Main Research Thrusts
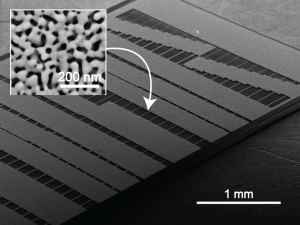
Nanostructured Electrochemical Biosensors
An integral component of a biosensor platform with an electrochemical read-out is the sensor element that interfaces biomolecular detection probes with instrumentation electronics. Sensor performance, on both biomolecular and electrical levels, is strongly influenced by sensor coating properties, such as morphology, surface chemistry, and metallurgy. While nanostructured materials have shown significant promise in enhancing the performance of these sensors, the underlying mechanisms for this enhancement are not fully understood.
Currently, we are fabricating novel nanostructured electrodes to provide insight into how nanoscale features enhance sensitive measurement and purification of nucleic acids in complex biological samples10-13. The fundamental studies will reveal a set of design rules for the development of nanostructured electrochemical sensor elements and assist technological advancements in food safety, water quality, and medical diagnostics.
Multifunctional Biomedical Device Coatings
There is a significant need for medical devices that can both monitor and modulate physiological activity, while minimizing adverse tissue response to an implant. This is particularly important in neurological disorders, since neurons exhibit both electrical and chemical activity as a part of their normal function and implanted neural interfaces typically suffer from deteriorating device performance due to tissue-material interactions.
Currently, we are creating multifunctional neural electrodes that incorporate tunable drug delivery and topographical cues. Specifically, we focus on the fundamentals of molecular release from nanoporous metals14-16 and cell-surface interactions as a function of nano-topography17. We expect that these efforts will translate into advanced neural interfaces for closed-loop control of neurological disorders such as epilepsy.

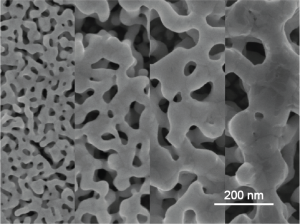
Libraries of Nanoporous Metal Morphologies
In the studies described above, nanoporous metal attributes (e.g., characteristic feature size, effective surface area, electrical conductivity, and surface chemistry) translate into several key device performance metrics: (i) limit of detection, sensitivity, and biofouling-resilience of affinity biosensors; (ii) biocompatibility, signal-to-noise ratio, and charge injection capacity of neural electrodes; and (iii) loading capacity, release kinetics, and on demand release in drug delivery platforms.
Currently, we are developing techniques to modulate pore morphology and surface chemistry with the goal of creating on-chip libraries that display multiple material attributes18-21. We expect these libraries to allow for high-throughput study of structure-property relationships in the context of biomolecule-tissue-material interactions, as well as other applications such as catalysis and energy storage.
Reference Papers
1 Seker, E., Gaskins, J., Bart-Smith, H., Zhu, J., Reed, M., Zangari, G., Kelly, R. & Begley, M. The effects of post-fabrication annealing on the mechanical properties of freestanding nanoporous gold structures. Acta Materialia 55:4593 (2007).
2 Seker, E., Reed, M. L. & Begley, M. R. A thermal treatment approach to reduce microscale void formation in blanket nanoporous gold films. Scripta Materialia 60:435 (2009).
3 Seker, E., Reed, M. & Begley, M. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2:2188 (2009).
4 Seker, E., Begley, M., Reed, M. & Utz, M. Kinetics of capillary wetting in nanoporous films in the presence of surface evaporation. Applied Physics Letters 92:013128 (2008).
5 Seker, E., Reed, M., Utz, M. & Begley, M. Flexible and conductive bilayer membranes of nanoporous gold and silicone: Synthesis and characterization. Applied Physics Letters 92:154101 (2008).
6 Huang, L., Seker, E., Landers, J., Begley, M. & Utz, M. Molecular Interactions in Surface-Assembled Monolayers of Short Double-Stranded DNA. Langmuir 26:11574 (2010).
7 Leslie, D., Easley, C., Seker, E., Karlinsey, J., Utz, M., Begley, M. & Landers, J. Frequency-specific flow control in microfluidic circuits with passive elastomeric features. Nature Physics 5:231 (2009).
8 Seker, E., Leslie, D., Haj-Hariri, H., Landers, J., Utz, M. & Begley, M. Nonlinear pressure-flow relationships for passive microfluidic valves. Lab on a Chip 9:2691 (2009).
9 Seker, E., Berdichevsky, Y., Begley, M., Reed, M., Staley, K. & Yarmush, M. The fabrication of low-impedance nanoporous gold multiple-electrode arrays for neural electrophysiology studies. Nanotechnology 21:125504 (2010).
10 Daggumati, P., Matharu, Z. & Seker, E. Effect of Nanoporous Gold Thin Film Morphology on Electrochemical DNA Sensing. Analytical Chemistry 87:8149 (2015).
11 Daggumati, P., Matharu, Z., Wang, L. & Seker, E. Biofouling-resilient nanoporous gold electrodes for DNA sensing. Analytical Chemistry87:8618 (2015).
12 Daggumati, P., Appelt, S., Matharu, Z., Marco, M. & Seker, E. Sequence-Specific Electrical Purification of Nucleic Acids with Nanoporous Gold Electrodes. Journal of the American Chemical Society 138:7711 (2016).
13 Zhou, J. C., Feller, B., Hinsberg, B., Sethi, G., Feldstein, P., Hihath, J., Seker, E., Marco, M., Knoesen, A. & Miller, R. Immobilization-mediated reduction in melting temperatures of DNA–DNA and DNA–RNA hybrids: Immobilized DNA probe hybridization studied by SPR. Colloids and Surfaces A: Physicochemical and Engineering Aspects 481:72 (2015).
14 Seker, E., Berdichevsky, Y., Staley, K. J. & Yarmush, M. L. Microfabrication-Compatible Nanoporous Gold Foams as Biomaterials for Drug Delivery. Advanced Healthcare Materials 1:172 (2012).
15 Kurtulus, O., Daggumati, P. & Seker, E. Molecular Release from Patterned Nanoporous Gold Thin Films. Nanoscale 6:7062 (2014).
16 Polat, O. & Seker, E. Halide-Gated Molecular Release from Nanoporous Gold Thin Films. The Journal of Physical Chemistry C 119:24812 (2015).
17 Chapman, C. A., Chen, H., Stamou, M., Biener, J., Biener, M. M., Lein, P. J. & Seker, E. Nanoporous Gold as a Neural Interface Coating: Effects of Topography, Surface Chemistry, and Feature Size. ACS Applied Materials and Interfaces 7:7093 (2015).
18 Chapman, C. A., Daggumati, P., Gott, S. C., Rao, M. P. & Seker, E. Substrate topography guides pore morphology evolution in nanoporous gold thin films. Scripta Materialia 110:33 (2016).
19 Chapman, C. A., Wang, L., Biener, J., Seker, E., Biener, M. M. & Matthews, M. J. Engineering on-chip nanoporous gold material libraries via precision photothermal treatment. Nanoscale 8:785 (2016).
20 Dorofeeva, T. S., Matharu, Z., Daggumati, P. & Seker, E. Electrochemically Triggered Pore Expansion in Nanoporous Gold Thin Films. The Journal of Physical Chemistry C 120:4080 (2016).
21 Dorofeeva, T. S. & Seker, E. Electrically tunable pore morphology in nanoporous gold thin films. Nano Research 8:2188 (2015).